Quote:
Originally Posted by sixto
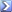
Those are variations of the ideal gas law. Can ideal gas laws apply to typical air at STP and STP + 2 bar?
Volume doesn't change, temperature doesn't chage (after equalizing) and the ideal gas constant doesn't change (if it even applies) so we're down to P1/n1 = P2/n2. P1 = 1 bar absolute, P2 = 3 bar absolute. If P2/P1 = 3, n2/n1 = 3. That's how I came up with 3:1 air mass ratio. But I don't know if all the stuff before getting to the P/n ratios is valid for a non ideal gas. Nor if a mole is directly proportional to mass in this situation.
Sixto
87 300D
|
Ideal gas law is a good first approximation. It breaks down when the gas in the sample is near a phase change.
The 'n' is the sum of the number of moles [6.02 * 10E24 molecules] for each different kind of molecule in the gas mixture [O2 + N2 + Ar + H2O + CO2 +....]